how many valence electron does sulfur have|10.6: Valence Electrons : Clark Ene 24, 2021 — How many Valence Electrons does Sulfur have? This is the reason that why sulfur may have either two or six electrons in its valency. The valency of sulfur is . Find Urologists in Ozamiz city with the help of our comprehensive guide and directory of Urologists. Use our database to search Urologists by name or specialty, and filter by location to find the best professional for you. . Urologists in ozamiz city Province of misamis occidental > Northern mindanao > Philippines. . 2nd floor manila .Get top player statistics, guides and more for the Ursa hero on Dotabuff, a Dota 2 stats platform.
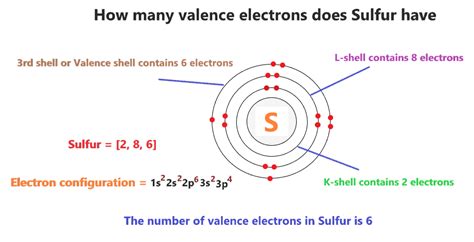
how many valence electron does sulfur have,Abr 17, 2023 — Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. .Consider sulfur's electron configuration, which was determined in the previous .
The total number of valence electrons for iron is 8: 2 electrons in the highest .May 28, 2024 — Consider sulfur's electron configuration, which was determined in the previous section and is replicated below. .
Ene 24, 2021 — How many Valence Electrons does Sulfur have? This is the reason that why sulfur may have either two or six electrons in its valency. The valency of sulfur is .Abr 1, 2024 — Sulfur's Valence Electrons: The Key to Chemical Behavior • Sulfur's Valence Electrons • Discover why sulfur's six valence electrons are crucial for its chemical .10.6: Valence Electrons Ago 17, 2020 — There are two ways to find the number of valence electrons in Sulfur (S). The first is to use the Periodic Table to figure out how many electrons Sulfur has in its .The number of electrons in a neutral atom is equal to the atomic number, or number of protons, in an atom. Valence electrons are the electrons which live in the outermost .Hun 17, 2014 — Sulfur has six valence electrons. Valence electrons are the outermost electrons which, therefore, are located on the highest energy levels. Consequently, .The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated .Abr 16, 2014 — The electron configuration for sulfur is: 1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (4) But sulfur has 6 valence electrons, tends to have a 2- charge and forms 2 bonds. However, .Mar 23, 2023 — For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence .
Mar 1, 2023 — In the case of sulphur, its electronic configuration of six valence electrons in the 3s and 3p subshells gives it a strong tendency to form covalent bonds with other atoms. How many valence electrons does sulphur have? Sulphur has six valence electrons, which are located in the 3s and 3p subshells.
Dis 15, 2019 — If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and .When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the Sulfur atom. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital.
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Mar 24, 2021 — 1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2. Arrange the atoms to show specific connections.Ene 24, 2021 — How many Valence Electrons does Sulfur have? This is the reason that why sulfur may have either two or six electrons in its valency. The valency of sulfur is subjective since it is up to the reaction of sulfur in the given scenario. You may read more about the variance of sulfur on the internet. It would provide you with decent exposure to .The atomic number of Sulfur S is 16. The electronic configuration of Sulfur S can be written as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4; The valence electrons are the sum of the electrons in the outermost shell, that is two 3 s electrons and four 3 p electrons which gives a total of six valence electrons. Therefore, the valence electron in a Sulfur S .how many valence electron does sulfur have 10.6: Valence Electrons From the electron configuration of neutral phosphorus atoms in Example \(\PageIndex{1}\), how many valence electrons and how many core electrons does a neutral phosphorus atom have? Solution The highest-numbered shell is the third shell, which has 2 electrons in the 3 s subshell and 3 electrons in the 3 p subshell.Peb 19, 2022 — The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron–electron repulsions are reduced. The entering electron does not experience as much repulsion and the chlorine atom accepts an .

Hun 27, 2024 — In its ground state, it corresponds to an atomic number that is unique to sulfur in the periodic table. . How many valence electrons does oxygen have? 6. Oxygen is in group 16 of the periodic table, so as with the other elements in groups 13-18, you can subtract 10 from the group number to find out the number of valence electrons.

How can you use the periodic table to find out how many valence electrons an element has? This article from Khan Academy explains the simple rules and patterns that can help you determine the number of electrons in the outermost shell of any atom. You will also learn why valence electrons are important for chemical bonding and reactivity.how many valence electron does sulfur haveHul 20, 2023 — Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration.The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. [1] Thus, the number of valence electrons that it may have depends on the electron .
The valence electrons are the electrons in the outermost electron shell of an atom.. That is why elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table.. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell, corresponding to the electron configuration #s^2p^6#.. This .
Abr 1, 2024 — Sulfur's Valence Electrons: The Key to Chemical Behavior • Sulfur's Valence Electrons • Discover why sulfur's six valence electrons are crucial for its chemi.You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons, respectively. Valence electrons are responsible for the reactivity of an element.Abr 16, 2014 — The electron configuration for sulfur is: 1s^(2)2s^(2)2p^(6)3s^(2)3p^(4) But sulfur has 6 valence electrons, tends to have a 2- charge and forms 2 bonds. However, there are exceptions.
how many valence electron does sulfur have|10.6: Valence Electrons
PH0 · What is the valence electron configuration of sulfur?
PH1 · Valence Electrons
PH2 · Sulfur Valence Electrons
PH3 · How to find the Valence Electrons for Sulfur (S)
PH4 · How many valence electrons does sulfur have?
PH5 · How many valence electrons are in an atom of sulfur?
PH6 · How Many Valence Electrons Does Sulfur (S) Have?
PH7 · 3.10: Valence Electrons
PH8 · 2.7: Applications of Electron Configurations: Valence
PH9 · 10.6: Valence Electrons